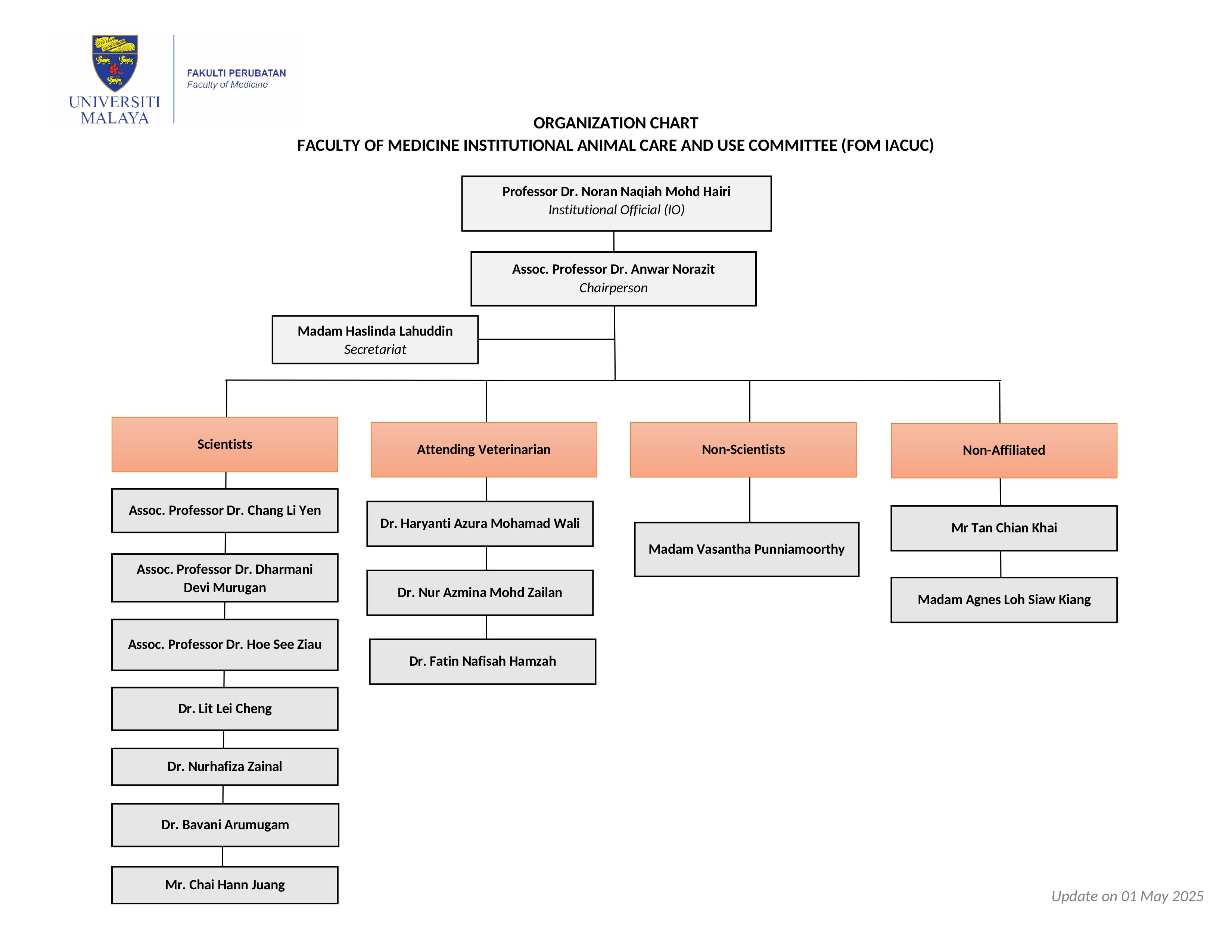
The Faculty of Medicine Institutional Animal Care and Use Committee (FOM IACUC) was established in the Faculty of Medicine in 2013. The FOM IACUC is responsible to provide oversight for the care and use of animals at the Faculty of Medicine. Any researcher/staff/student who intends to conduct work, which involves the use of animals in the animal facility located in the Faculty of Medicine, must submit an Animal Use Protocol (AUP) application to FOM IACUC. The FOM IACUC reviews the AUP application and approves it before the animal work is allowed to commence. To date, more than 300 applications have been submitted to the FOM IACUC by researchers/staff/students from UM, as well as from outside UM for review and approval.
The FOM IACUC will:
| Forms | Link |
| 1. Meeting Schedule 2026 | IACUC Meeting 2026 & Deadline of Submission |
| 2. Animal Use Protocol (AUP) Application Form | FOM IACUC Animal Use Protocol (AUP) 2023_v4_20231102 |
| 3. Animal Use Protocol (AUP) Amendment Form | FOM IACUC AUP Amendment Form_v2_20231102 |
| 4. How to Apply Animal Ethics Protocol | Animal Ethics Processing Fee |
1. UM FOM IACUC Policy_v2_20181108
2. Guide Administration Substances Removal Blood
3. Acute Oral Toxicity – Acute Toxic Class Method 423
4. Anesthesia of Other Lab Species
5. Euthanasia of Other Lab Species
6. Guidelines for the welfare and use of animals in cancer research
7. Toxicokinetics_Intravenous toxicity_OECD417
For more information, please contact 03-7967 7515 / fomiacuc@um.edu.my
Last Update: 31/12/2025