We used to learn that our brain cells stopped dividing with age and that our brains are thus static, hence there will be limited chances of recovery after any injury to our adult brains. However, this is no longer true. The concept of neuroplasticity is now often quoted in neuroscience and neurorehabilitation. In simple terms, neuroplasticity can be described as the brain’s neural network’s ability to change, reorganise and adapt to changes such as experience, stimulation or pathology.

In rehabilitation medicine, this concept is used to encourage recovery from stroke or brain injuries, via the concept of specific practice, high repetition and intensity. For example, a patient with adequate motor recovery is prescribed with robotic exoskeletons to deliver the high number of steps/minute repetition to enhance neuroplasticity. When coupled with an enriched environment of adequate stimulation and novelty, with achievable but challenging tasks, such rehabilitation program can facilitate further synaptic re-connections. This concept allows incorporation of virtual reality, interaction and gamification into rehabilitation as well.
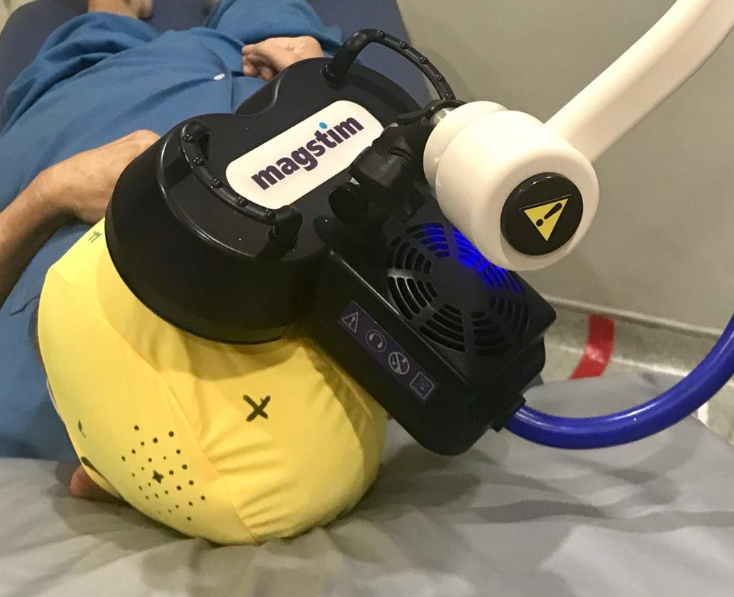
On top of the behavioural approaches, direct neural stimulation with non-invasive brain stimulation (neuromodulation) is also being investigated to facilitate neuroplasticity. As our neurons function via an electrical gradient across the cell membrane, using an external physical agent can influence the brain’s cellular function. An example is the Transcranial Magnetic Stimulation (TMS) procedure which induces an electrical counter current in the cortical neurons below the coil when applied on the cranium. At submaximal power, a repetitive TMS (rTMS) can stimulate or inhibit those neurons. Therefore, rTMS can prime the brain neural network for adapting and relearning and further augment the effects of behavioral rehabilitation therapy.
Another neuromodulatory approach is applying a small constant electrical current (2 miliAmp) via two sponge electrodes on the skull. This is termed Transcranial Direct Current Stimulation (tDCS). The cortical neurons under the anode electrode are excited whilst that under the cathode is inhibited. Combined with behavioural therapy, this procedure may enhance learning. We have found that both 5-Hz rTMS and anodal tDCS induced effects on corticospinal excitability in persons with chronic stroke lasting at least 1 hour after stimulation (1). Our latest research project is to investigate the role of TMS to obtain the lower limb motor evoked potentials, which can potentially guide the neurorehabilitation of gait impairment after stroke. This project is funded by a Fundamental Research Grant Scheme grant with co-researchers in the Faculty of Engineering (2).
References:
1. Goh, H. T., Chan, H. Y., & Abdul-Latif, L. (2015). Aftereffects of 2 noninvasive brain stimulation techniques on corticospinal excitability in persons with chronic stroke: a pilot study. Journal of neurologic physical therapy : JNPT, 39(1), 15–22.
2. Mapping the corticomotor pathway of different lower limbs musculature from deep penetration transcranial magnetic stimulation responses. FRGS (FP002-2020)
For more on translating neuroscience, technology or neuromodulation into clinical practice, do get in touch with us at kj_rhm@ummc.edu.my